Organ-on-chip technology can outperform animal models and could generate billions in increased R&D productivity, according to the outcomes of a study published in Communications Medicine (December 2022).
For “Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology,” Lorna Ewert, et al, analyzed 870 Liver-Chips to determine their ability to predict drug-induced liver injury caused by small molecules identified as benchmarks by the Innovation and Quality consortium.
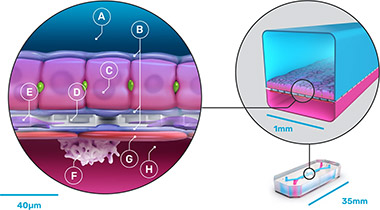
The chips were made from flexible polydimethylsiloxane (PDMS), a transparent viscoelastic polymer material, with compartmental chambers consisting of two parallel microchannels separated by a porous membrane containing pores of 7 µm diameter spaced 40 µm apart. Cultured human hepatocytes were sandwiched within an extracellular matrix on a porous membrane within the upper parenchymal channel. Human liver sinusoidal endothelial cells, Kupffer cells, and stellate cells were cultured on the opposite side of the membrane in the lower vascular channel.
The 870-chip experiment was carried out in five consecutive cycles to test a selection of 27 drugs at varying concentrations relative to the average therapeutic human Cmax obtained from literature. The researchers also developed an economic model to assess the impact of improvements in the predictive validity of preclinical toxicology models on the economics of drug development.
They found that the Liver-Chip met the qualification guidelines across a blinded set of 27 known hepatotoxic and non-toxic drugs with a sensitivity of 87% and a specificity of 100%. “Said differently, the Liver-Chip detected nearly 7 out of every 8 drugs that proved hepatoxic in clinical use despite having been deemed to have an appropriate therapeutic window by animal models,” the authors wrote. “The Liver-Chip similarly detected 2 out of 4 such drugs that were additionally missed by 3D hepatic spheroids. We therefore believe that these findings advocate the routine use of the human Liver-Chip in drug discovery programs to enhance the probability of clinical success while improving patient safety.”
The economic model revealed that this rate of performance could generate over $3 billion annually for the pharmaceutical industry through increased small-molecule R&D productivity.







