First emerging in China in December 2019, the novel coronavirus, SARS-CoV-2, quickly evolved into a full-fledged global pandemic by March 2020, creating unprecedented pressure for the global healthcare industry. The rapid spread of COVID-19 brought to the fore the significance of a robust healthcare infrastructure, most notably in terms of diagnostics and testing capabilities, in turn amplifying the demand for advanced COVID-19 detection kits and tests.
Organizations such as the National Institutes of Health (NIH) implemented wide-ranging collaborative initiatives, including the Rapid Acceleration of Diagnostics (RADx) Initiative in early 2020, to encourage the development of testing and vaccine strategies. Taking heed of this, several researchers and healthcare entities have been making efforts to ramp up the development of advanced diagnostics tests to facilitate quick and precise detection of the COVID-19 virus among populations worldwide. Abbott is a prime example, having delivered more than 300 million COVID-19 tests across the globe as of December 2020.
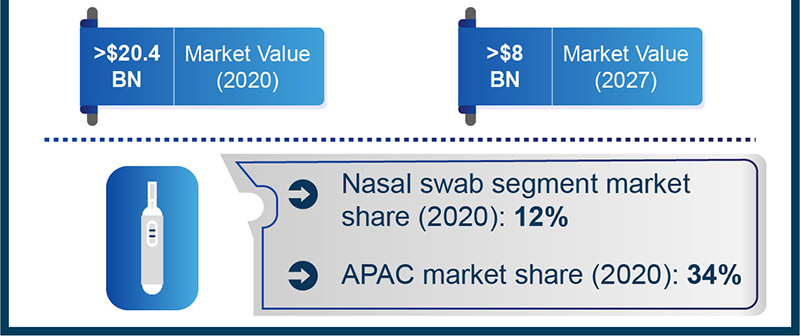
Diagnostic advances bode well for the imminent expansion of the global COVID-19 detection kits market, which is expected to be worth $8 billion by 2027, according to estimates from Global Market Insights, Inc.
Technological Advancements Trigger Transformation in COVID-19 Diagnostics
In recent months, the introduction of several COVID-19 vaccine candidates with high efficacy levels has emerged as a crucial milestone in the fight against the pandemic, with the drive for widespread inoculation coming into effect worldwide. Even so, key organizations in the healthcare domain continue to promote COVID-19 testing as an important safety measure, particularly when conducted in tandem with the vaccination initiatives. For instance, a recent survey by The Harris Poll revealed that nearly 82% of Americans considered testing to be an important partner to a successful vaccine rollout. As such, the advancement of novel COVID-19 detection technologies has become a key point of interest for many major entities in the global healthcare sector.
While molecular techniques such as RT-PCR (reverse transcription polymerase chain reaction) are considered to be the highest standard in early virus detection, limitations pertaining to their bedside testing application are coming to light recently, owing mainly to the technical complexities associated with the protocol. Consequently, many POC (point-of-care) assays have gained traction as a viable solution to these challenges, given their ability to facilitate faster COVID-19 diagnostics outside conventional testing lab settings.
At the current stage of the coronavirus pandemic, point-of-care testing kits, such as lateral flow immunoassays (LFIA), are showing immense potential in timely detection of COVID-19. They boast higher approachability from users, greater sensitivity and accuracy, and ease of use, all of which could help alleviate some of the testing burdens on central hospitals. This rapid surge in popularity of LFIAs has, in turn, triggered the demand for associated testing equipment, especially immunoassay test strips, which accounted for nearly 29% of the COVID-19 detection industry share in 2020, as per GMI reports.
In October 2020, Siemens Healthineers introduced an easy-to-use and rapid antigen testing kit for SARS-CoV-2 testing. Dubbed the CLINITEST Rapid COVID-19 Antigen Test, the POC cassette test is designed to be administered via the nasopharyngeal swab method, without the need for specialized laboratory personnel or equipment, and provides results within 15 minutes.
As the world grows increasingly more digitized, next-gen technologies such as artificial intelligence are demonstrating strong potential in various advanced fields, including medical imaging and diagnostics. The use of AI has been successfully implemented for a plethora of critical medical applications, gaining particular traction during the ongoing pandemic, for disease cluster identification, case monitoring, COVID-19 diagnostics, prediction of looming outbreaks, disease pattern recognition, record maintenance, training, and more. Such is the potential of artificial intelligence in advancing medical technology, that it is quickly becoming inculcated as a major part of COVID-19 response measures worldwide, including as a solution for coronavirus testing.
Last February Laipac Technology Inc. collaborated with UAE-based firms Pure Health LLC and YAS Pharmaceuticals, LLC to leverage the power of technology and introduce the first AI-powered rapid COVID-19 antigen test system in the world. The European CE-IVD certified COVID-19 testing kit, called the LooK SPOT AI COVID-19 Antigen Rapid Test System, is a smartphone-powered lateral flow immunoassay diagnostic device designed to facilitate qualitative nucleocapsid protein detection from SARS-CoV-2 via nasal swabs.
Europe Takes Point on Coronavirus Testing with Favorable Regulatory Framework
While every region across the globe is undertaking varying degrees of effort to strengthen themselves against the onslaught of the ongoing pandemic, Europe has emerged as one of the most prominent in recent times, especially with regards to coronavirus testing and contact tracing initiatives. The EU bore a major brunt of the crisis. Significant infection-related adverse effects across numerous patient populations and widespread fatalities across all major European territories, including Italy and the United Kingdom, in turn driving up the need for effective and rapid SARS-CoV-2 testing in the region.
GMI research suggests that Europe accounted for nearly 35% of the COVID-19 detection kits market in 2020, owing to burgeoning investment interest in making COVID-19 testing kits easily available to patients. In October 2020, the EU mobilized funds of nearly €100 million for the procurement of rapid antigen testing kits to be delivered across EU nations using the Emergency Support Instrument.
Additionally, EU regulatory authorities have also begun to award CE certifications to emerging COVID-19 detection kits at a faster rate, in order to encourage self-testing within Europe, using authorized kits.
For instance, in June Abbott received the CE Mark for its Panbio COVID-19 Antigen Self-Test diagnostics and it was authorized for direct sale to consumers. The self-testing kit, based on Abbott’s proprietary lateral flow technology, makes use of minimally invasive nasal swabs and is designed to be administered sans instrumentation, for detection of COVID-19 in symptomatic as well as asymptomatic adults and children within 15 minutes.
A robust and efficient testing infrastructure is essential, and COVID-19 detection kits are increasingly considered indispensable tools for tracking, and eventually ceasing, the transmission of the SARS-CoV-2 virus over the years ahead. Although most people believe the crisis can be addressed gradually and the world will go back to “normal”, the risk and presence of the virus is expected to be prevalent post-pandemic as well.